Our EBM Glossary includes commonly-used terms in scientific research and our calculators’ Evidence Appraisal sections. To help categorize these terms, we’ve added icons for Function to show the area of research the term is most relevant for (risk, diagnosis, treatment, prognosis, any/all) and Stage to show where along the process of evidence generation it’s generally used (study design, study question, data collection, primary data analysis, secondary data analysis).
Definition: Probability of an outcome.
Example: Using the ASCVD Risk Calculator we determined that a 50 year old African-American diabetic male nonsmoker with a total cholesterol of 300 mg/dL, HDL 80md/dL, and systolic BP 120, and not on hypertension medication has an 8.6% absolute risk of a cardiovascular event in the next 10 years, meaning that out of 100 patients like him, about 9 of them will have nonfatal MI or stroke, or die from MI or stroke. According to the ASCVD study, his absolute risk could be reduced to 3.9% if his risk factors were optimized (e.g. control diabetes, start statin). Absolute risk reduction/increase (ARR/ARI)
Definition: Difference in absolute risk between the control group and the tested group OR exposed and unexposed population.
ARR/ARI = |Group A risk - Group B risk| Also called: absolute risk difference
Example: Using the ASCVD Risk Calculator we determined that a 50 year old African-American diabetic male nonsmoker with a total cholesterol of 300, HDL 80, and systolic BP 120, and not on hypertension medication has an 8.6% absolute risk of a cardiovascular event in the next 10 years. According to the ASCVD study, his absolute risk could be reduced to 3.9% if his risk factors were optimized (e.g. control diabetes, start statin).
For the patient above, by optimizing risk factors his absolute risk reduction would be 4.7%.
Pitfall: A related but different way of quantifying risk reduction is the relative risk reduction (RRR). For this patient, the RRR would be a whopping 54.7%. Drug and device manufacturers often quote the RRR of their treatment instead of the absolute risk reduction. Definition: Altering the absolute risk based on additional information (i.e., history, diagnostic tests, demographic information). Example: Younger patients tend to have better prognosis than older patients. How much better? HCT-CI creator, Mohamed Sorror, studied the impact of age on prognosis in patients with hematologic cancers and added an adjustment of 1 point for patients 40 years or older, thus creating the age-adjusted HCT-CI. Definition: Manipulation of study design or results to achieve a favored outcome. May be intentional or unintentional.
Definition: Hiding the intervention from the patients and those interpreting the results. A study may be blind (patient doesn’t know what treatment they’re getting), double-blind (doctor and patient don’t know what treatment they’re getting), or triple-blind (statistician analyzing data doesn’t know what treatment was given).
Also called: blind, double-blind, or triple-blind.
Use case: Method to remove bias from a study.
Definition: Study that compares a series of patients with the same diagnosis. No control group.
Use case: Compare outcomes across patients of the same diagnosis with varying demographics and treatments. If you’re an obstetrician and you noticed that you’ve delivered several babies in a row with birth defects whose mothers took the same morning sickness medication, you might publish a case series with information about the pregnancy and the mother in order to start looking for a cause. (This is the story of thalidomide.)
Definition: Compares a group with the disease to one without the disease.
Use case: Evaluate the relationship between an attribute and the disease. If you were trying to figure out if cigarette smoking is associated with lung cancer, you might start by looking at patients who have lung cancer and patients who don’t have lung cancer, and note how many smokers are in each group. Spoiler alert: this has already been done multiple times since the 1940s.
Definition: Attribution of causes for an effect. There are two types of causes: 1) a necessary cause must come before the effect, and 2) a sufficient cause occurs before the effect but isn’t the only required cause.
Definition: A measure used to describe the center of data: mean, median, and mode.
Definition: A difference that is significant enough to impact patient outcomes. Statistical significance does not always imply clinical significance. Example: A drug that lowers hemoglobin A1c by 1% might be statistically signficant, but may not be clinically significant if it doesn’t improve outcomes like cardiovascular death, diabetic nephropathy, or diabetic retinopathy.
Definition: A study that compares a control group who didn’t receive the intervention or exposure with a group who did receive the intervention or exposure, so incidence rates can be compared. Commonly done with large groups over long periods of time.
Definition: An interval within which you can be confident (usually 95% confident) that the true value lies.
Definition: A variable that affects the outcome but isn’t the variable you are interested in.
Definition: The relationship between two variables.
Definition: A study that collects data on both exposure and outcomes at a single point in time.
Definition: A study where subjects are exposed to a sequence of two or more treatments.
Definition: The variable that is dependent on the effect of the independent variable.
Dose-response relationship
Definition: A relationship where varying the dose alters the outcome (i.e., a bigger dose has a bigger effect than a smaller dose).
Experimental event rate (EER)
Definition: Measure of patients in the experimental group who experience an event.
Definition: Validation that the study’s theorized cause and effect can be applied to a broader population.
Definition: Graphical representation of study outcomes in a meta-analysis.
Example: 
Definition: Scatterplot of outcomes for individual patients compared with expected results. Used in a meta-analysis to shed light on any bias.
Definition: The most widely accepted standard for diagnosis, measurement or evaluation.
Also called: reference standard and criterion standard
Definition: Improved outcomes caused by the patient knowing that they are being studied.
Also called: sentinel effect
Example: Studies related to handwashing and hygiene had to account for the Hawthorne effect, because people who knew they were being observed were more likely to be compliant.
Definition: The amount of variation or incompatibility in studies that are part of a systematic review.
Definition: A graph that represents the frequency of a variable.
Example: 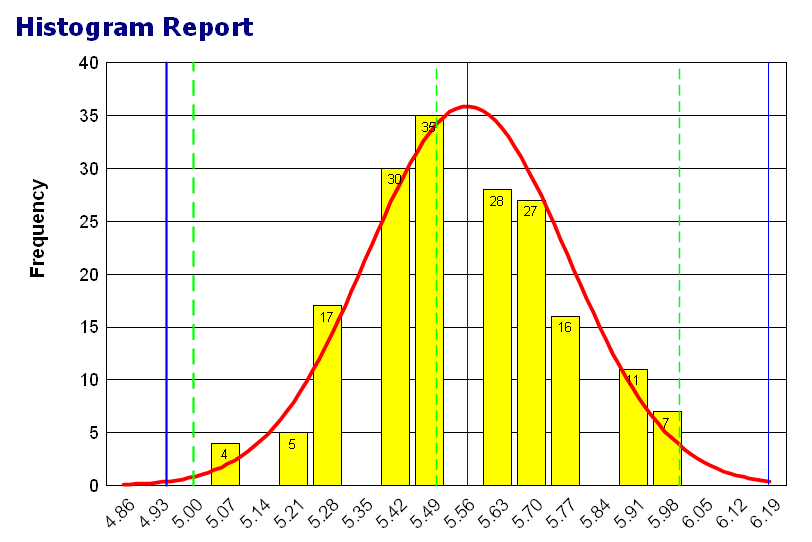
Definition: How many cases occur over a defined period of time.
Pitfall: Sometimes confused with prevalence, which is the number of cases present at a single time point. Definition: The measure of a test against the current gold standard.
Intention to treat analysis
Definition: Analysis of subject in the groups that they were originally assigned, even if they crossed over, dropped out or died. This is done to minimize bias.
Definition: Validation that the study’s theorized cause and effect are accurate for the study subjects. “Is my theory proven in this particular group of patients I’ve studied?”
Pitfall: Evidence that is only internally validated should be applied with caution in clinical practice.
Definition: The middle 50%, or the range from 25th to 75th percentile.
Example: 
Kaplan-Meier survival graph
Definition: A graph of chances of an outcome over time. The number of subjects without an outcome divided by the number with the outcome at various time intervals. Usually more accurate at the earlier time intervals than towards the end.
Also called: Kaplan-Meier curve, survival curve
Example: 
Definition: Likelihood of a given test result in patients with a diagnosis compared to patients without the diagnosis.
Definition: A predictive graph used to identify outcomes when there are multiple independent variables.
Example:

Medical subject headings (MeSH)
Definition: Glossary, often used by papers and databases to define medical terms.
see also:
Definition: The statistical process of combining data from multiple independent studies focused around the same question.
Multiple linear regression
Definition: Graphical representation of an outcome based on one dependent variable and two or more independent variables.
Example: 
Definition: A study with only one patient.
Negative predictive value (NPV)
Definition: The probability of a negative test result when a disease is absent (i.e., how accurate a negative result is).
Definition: Qualitative or categorical data. Cannot be put into an order.
Example: Colors: red, green, and blue.
Definition: The bell curve.
Example:

Definition: A hypothesis that there is no difference between two variables.
Number needed to harm (NNH)
Definition: The number who need to be treated in order to cause one bad outcome. It is equal to 1/ARI.
Number needed to treat (NNT)
Definition: The number of patients who need to be treated in order to cause one good outcome. It is equal to 1/ARR.
Definition: Study that occurs without intervention by the investigator.
Definition: Ratio of the measured outcome for the treated group and control group.
Definition: A test to determine the null or alternative hypothesis. If the outcomes fall to one side of the normal distribution then the alternative hypothesis is accepted.
Definition: Data that can be ordered but also has a qualitative category.
Example: Temperature: cold, colder, coldest
Definition: A study that measures outcomes over a time interval.
Definition: The probability that an outcome is caused by chance.
Patient expected event rate (PEER)
Definition: The expected rate for an outcome in an individual who receives the standard or no treatment.
Also called: expected event rate (EER)
Patient-oriented evidence (POE)
Definition: Evidence that pertains to areas that patients care about (e.g. symptoms, quality of life, mortality, etc), as opposed to clinical benchmarks like percent reduction of HgbA1c or decrease in systolic blood pressure points.
Permuted block randomization
Definition: Strategy for further randomization. Patients are assigned to different treatment options for a block of time and then a different treatment option for the next block of time.
Positive predictive value (PPV)
Definition: The probability of a positive test result when a disease is present (how accurate a positive test result is).
Post-marketing surveillance
Definition: A process for evaluating outcomes and side effects for a medication after it is available to the public.
see also:
Definition: How likely it is that a patient has an outcome (after being tested).
Definition: The ability of a study to measure any statistically significant difference (if any exists).
Definition: How likely it is that a patient has an outcome (before being tested). Often synonymous with prevalence.
Definition: How many cases are present at a single time point.
Pitfall: Sometimes confused with incidence, which is the number of cases that occur over a defined period of time. Definition: Single/individual studies based on original data, as opposed to secondary research, which synthesizes the evidence from primary research. Definition: Study designed to measure outcomes that have not yet occurred.
Also called: cohort study or longitudinal study
Definition: The decision to publish (or not) based on the outcomes of the study.
Randomized controlled trial
Definition: A trial where patients are randomly assigned to either the treatment or control group.
Receiver operating characteristic (ROC) curve
Definition: A graph of sensitivity vs (1 - specificity). Used to determine cutoff points and evaluate the quality of a study. An area under the ROC (AUROC) of 0.8 is generally considered good.
Definition: Using one or more independent variables to measure or predict the dependent variable or outcome.
Definition: A line drawn between variables on a graph to predict an outcome.
Definition: Probability that an event will occur in the exposed group divided by probability it will occur in the unexposed group.
Relative risk reduction (RRR)
Definition: Relative change in risk between the control and treatment groups.
RRR = (Group A risk - Group B risk)/Group A risk x 100%
Definition: A study that is based on outcomes that have already occurred.
Definition: Ratio for risk between the treated and control groups.
Definition: The size of the sample or study participants. In general, a bigger sample size is better.
Definition: Bias introduced when sampling.
Definition: A synthesis of single/indvidual studies (primary research), such as a systematic review. Definition: Bias that occurs when choosing individuals for a study.
Definition: The probability of a positive test result when a disease is present.
Definition: When the sensitivity of a tool is good enough to rule out a disease based on a negative result. Definition: When the specificity of a tool is good enough to rule in a disease based on a positive result. Definition: The probability of a negative test result when a disease is absent.
Definition: The amount of variation in data points for a group. The implication of a high standard deviation (i.e., lots of variation in data) is that the data are less reliable.
Calculated by 1) finding the deviation for each value (subtract each data point from the average and square it), 2) finding the average variance (find the average deviation), and 3) taking the square root of the variance.
Example:

Standard error of estimate
Definition: The measurement of the accuracy of predictions that are made using a regression line. Definition: The measure of confidence that an intervention was the cause for an altered outcome. A statistically significant result is one that you are fairly sure, according to your analysis, happened because of your intervention, and not by chance.
Definition: How accurately an instrument measures data.
Definition: A technique that uses regression lines to find variables or subsets of variables that most accurately predict an outcome. Definition: The probability that a difference in outcome between treatment and control groups can be attributed to the treatment.
Definition: The process of designing a clinical trial. Several factors, including risk, bias, and study participants, dictate the study design.
Definition: How accurately a study measures an outcome.
Surrogate outcome/endpoint
Definition: Measures an endpoint in place of measuring the clinical benefit when it is not possible to measure benefit.
Pitfall: Surrogate outcomes are sometimes used to act upon data, perhaps prematurely.
Definition: A review of the literature around a specific research question. Similar, but not equivalent, to a meta-analysis, which refers to the process of combining data (i.e., a systematic review may include a meta-analysis, but not every meta-analysis is necessarily part of a systematic review).
Pitfall: Often used interchangeably with meta analysis (erroneously). Definition: When a negative test indicates absence of a disease.
Definition: When a positive test indicates the presence of a disease.
Definition: A test used when the alternative hypothesis is nondirectional (could fall on the negative or positive side of the null hypothesis).
Definition: An error made by accepting the alternate hypothesis when it is actually incorrect, e.g. imprisoning an innocent person.
Definition: An error made by accepting the null hypothesis when it is actually incorrect, e.g. letting a guilty person go free.
Definition: When patients that have a negative test result are not measured using the gold standard.